What Is The Oxidation Number Of Sulfur In Sodium Sulfate Na 2so 4
20032020 Therefore the oxidation number of sulfur is 4 it lost four electrons to oxygen and the oxidation numbers for our compound is as follows. Calculate oxidation number of sulphur from is as follows.

Pdf The Effect Of Addition Of Sodium Sulphate Na2so4 To Nickel Slag Pyrometallurgical Process With Temperature And Additives Ratio As Variables
Fluorine in compounds is always assigned an oxidation number of -1.
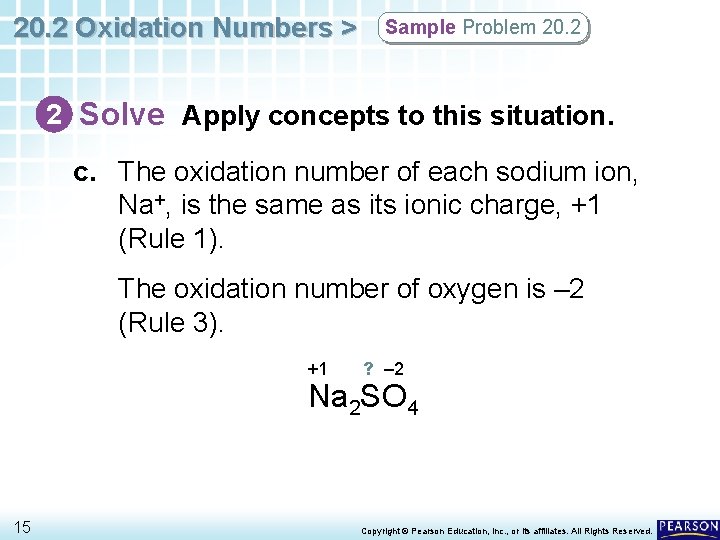
What is the oxidation number of sulfur in sodium sulfate na 2so 4. You can also do this algebraically by setting up an equation equal to zero. Therefore the oxidation number of sulphur in is 25. S VI The oxidation numbers of the most important compounds of sulfur.
Kaneppeleqw and 9 more users found this answer helpful. 19022021 Question 121 The following data are obtained when dinitrogen and dioxygen react together to form different compounds. 16052019 Sodium thiosulfate is a compound of sulfur used to develop photographs.
H X 2 S O X 5 has an oxygen-oxygen bond. Let the oxidation state or number of sulfur in sulfuric acid H 2 SO 4 x. The oxidation number of a free element is always 0.
The oxidation number of a monatomic ion equals the charge of the ion. H 2 SO 4. The oxidation state of sodium in N a H g is zero because it is a fused state of sodium in mercury which is a free state there in a free state the oxidation state of any element always zero.
What is the oxidation number of sulfur in Na 2 S 2 O 3 5H 2 O. As shown in. Therefore 21 x 4-2 0.
Sodium Sulfate Anhydrous is the anhydrous sodium salt form of sulfuric acidSodium sulfate anhydrous disassociates in water to provide sodium ions and sulfate ions. Since the sum of the oxidation numbers of all types of atoms in a compound must be zero that of sulfur must be. Sodium sulfate anhydrous is an electrolyte replenisher and is used in isosmotic.
E3radg8 and 78 more users found this answer helpful. Rules for assigning oxidation numbers. 19032020 Subtract the summed value from the overall charge of the compound.
The oxidation number of the sulfur in. Also do you count the numbers. Therefore the oxidation number of sulfur is 4 it lost four electrons to oxygen and the oxidation numbers for our compound is as follows.
Hydrated sodium thiosulfate has the formula Na 2 S 2 O 3 5H 2 O. The alkali metals group I always have an oxidation number of 1. The charge of SO4 sulfate is 2-.
ΔrH0 90 kJ mol1 NO g 1 2 O2 g NO2 g. Similarly what is the oxidation state of S in na2so3. Initial concentrations were 05-08 ppm as sulfite-sulfur.
This means that two of the five oxygen atoms have an oxidation number of 1. 04012010 There is no oxidation number for compounds only the elements within compounds. 19112018 It has 4 electrons in the 4p shell.
Metal ions ion in a coordination compound possesses two kinds of valency like primary and secondary valency. In hydrogen sulfide and pyrite the element sulfur is present in a reduced form in the other compounds it is oxidized. Hence the typical oxidation number of the sulfide ion is -2.
Same case as in H X 2 O X 2. Or x 6. The answer on the mark scheme is 2 but i dont understand how you get to that.
11022018 Oxidation state of oxygen is -2 so the oxidation state of six oxygen atoms will be -2. 2 I 4 -II -VI ie. For example Na2SO4 Na2 yields 2 plus 1 4 minus 2 minus 6.
Sodium ion is the principal cation of the extracellular fluid and plays a large part in the therapy of fluid and electrolyte disturbances. 1 Sodium sulfite was dissolved in the deionized water containing some metal salts and EDTA 4 Na salt. Let the oxidation state of sulphur is x.
The alkaline earth metals group II are always assigned an oxidation number of 2. Answer verified by Toppr. Therefore the oxidation number of sulfur is 4 it lost four electrons to oxygen and the oxidation numbers for our compound is as follows.
Metals in coordination compounds. The oxidation number of each oxygen is 2-. 04032019 oxidation number is 1.
Sulfide ion is formed at the lowest oxidation state that sulfur can achieve. ΔrH0 74 kJ mol1 If not in which direction. This makes sense because if the two sodium atoms gave a total of two electrons and the total oxidation number of oxygen there are three of them is -6 it gained six electrons then the remaining four electrons must have come from sulfur.
2 1 1 x 3 2 2 1 0 2 x 6 2 0 x 6. Since losing 4 electrons requires a lot of energy sulfur will gain the 2 electrons in the 4p shell and will have a valency of -2. DISCUSSION The mechanism of oxidation of sulfite in water In dilute solution sulfite is slowly oxidized to sulfate.
Mass of dinitrogen Mass of dioxygen 21 Comment on the thermodynamic stability of NO g given 1 2 N2 g 1 2 O2 g NO g. I know its -2 for oxygen and 1 for hydrogen but how do i work our the rest. 2 4x - 12 0.
Calculate The Oxidation Number Of Each Sulphur Atom In The Following Compounds Studyrankersonline

Pdf The Effect Of Addition Of Sodium Sulphate Na2so4 To Nickel Slag Pyrometallurgical Process With Temperature And Additives Ratio As Variables

Determine The Oxidation Number Of Sulphur In Na2so4 Brainly In

Is Na2so4 Sodium Sulfate Ionic Or Covalent Youtube
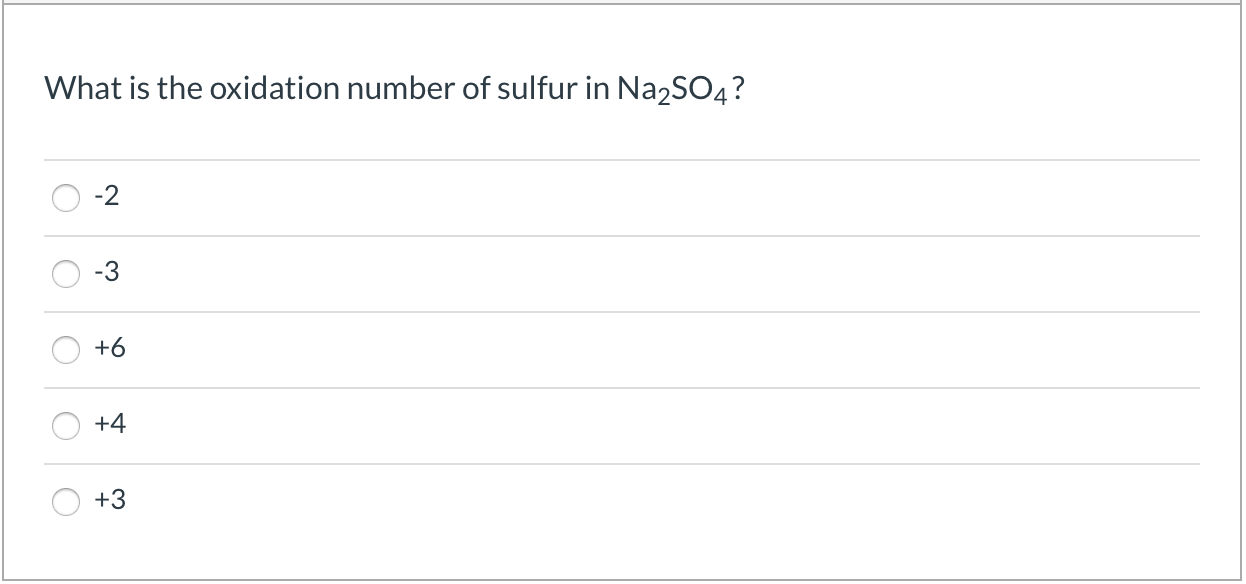
Solved What Is The Oxidation Number Of Sulfur In Na2so4 Chegg Com
Komentar
Posting Komentar